Introduction of Aluminum Plate Oxidation
Aluminum plate is a versatile and widely used metal plate due to its lightweight nature and excellent corrosion resistance. However, one of the challenges with aluminum is its tendency to oxidize when exposed to oxygen and moisture. Aluminum plate oxidation can lead to surface discoloration, loss of shine, and even structural degradation. To ensure the longevity and aesthetics of aluminum plates, preventive measures must be taken. In this blog post, we will explore six effective methods to prevent aluminum plate oxidation, providing you with practical solutions to protect your aluminum surfaces.

Protective Coatings
Applying protective coatings is one of the most common methods to prevent aluminum plate oxidation. These coatings act as a barrier between the aluminum surface and the surrounding environment, effectively shielding it from oxygen and moisture. Clear lacquers, paints, anodizing, and powder coatings are popular options for protective coatings. Clear lacquers and paints provide a transparent layer that seals the surface, while anodizing involves creating a controlled oxide layer on the aluminum surface. Powder coatings offer a durable and resistant barrier against oxidation. By choosing the appropriate protective coating, you can significantly enhance the resistance of aluminum plates to oxidation.

Anodizing
The electrochemical process known as anodizing creates a protective oxide layer on the surface of aluminum plates. By generating a thicker and more resilient oxide layer, this method improves aluminum’s inherent corrosion resistance. In addition to having excellent oxidation resistance, anodized aluminum surfaces also have increased scratch resistance and may maintain brilliant hues. The thickness and characteristics of the oxide layer can be customized to meet specific requirements by adjusting anodizing process parameters such voltage, electrolyte content, and time. In fields including architecture, automotive, and aerospace, anodizing is a common technique to stop aluminum plate oxidation.

Passivation
Passivation includes the use of a passivating agent, generally in the form of a chemical solution, to treat aluminum surfaces. This treatment effectively eliminates impurities and contaminants, thereby facilitating the development of a thin, protective oxide layer. The process of passivation improves the aluminum plates’ ability to stand oxidation by offering a stable shield against environmental elements. It proves particularly valuable in industrial applications where the prevention of surface discoloration and corrosion is crucial. Following passivation, it is advisable to rinse and dry the aluminum plates thoroughly to ensure the complete removal of any residual chemicals. Regular passivation treatments can significantly prolong the lifespan of aluminum surfaces.
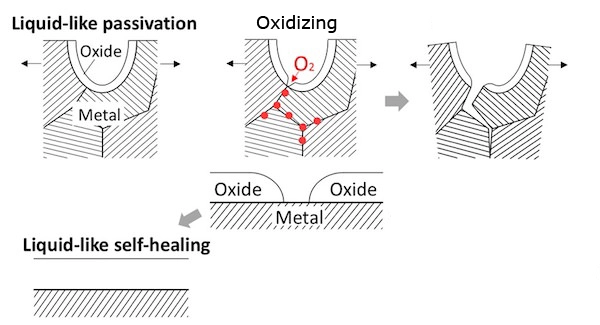
Protective Films
Protective films offer a temporary barrier against oxidation for aluminum plates. These films, made of plastic or adhesive materials, are applied to the surface to prevent direct contact with oxygen and moisture. Protective films are commonly used during transportation, storage, or fabrication processes to safeguard aluminum plates from scratches, stains, and oxidation. Once the plates are ready for use, the protective film can be easily removed, revealing a pristine, oxidation-free surface. This method is particularly useful when handling large quantities of aluminum plates or during construction projects where extended protection is required.

Proper Storage
Proper storage is crucial for preventing aluminum plate oxidation. Storing aluminum plates in a controlled environment can significantly reduce their exposure to moisture and oxygen. It is essential to store aluminum plates in a dry and low-humidity area, away from direct sunlight and sources of moisture. Utilizing storage cabinets, racks, or containers that provide airtight seals can further enhance the protection against oxidation. Additionally, ensuring proper ventilation and air circulation in the storage area helps to maintain optimal conditions and prevent the buildup of moisture that could lead to oxidation.

Avoiding Contact with Reacting Substances
Aluminum plates should be kept away from substances that can accelerate oxidation, such as acidic or alkaline solutions. These chemicals can initiate corrosion reactions that promote the oxidation process. It is crucial to handle aluminum plates with clean, non-reactive gloves and tools to prevent contamination. When cleaning aluminum surfaces, use mild, non-abrasive cleaners that are specifically formulated for aluminum. By avoiding contact with reactive substances and employing appropriate cleaning practices, you can minimize the risk of oxidation and maintain the integrity of aluminum plates.
Common aluminum alloys with good corrosion resistance
- Aluminum Alloy 6061: This particular aluminum alloy shows outstanding rust resistance, especially when it comes to standing chloride and nitrate corrosion. It finds extensive uses in industries such as aerospace, automotive, and structural engineering. By the way, if the requirements for corrosion resistance are not high, 3003 aluminum plate can also provide good corrosion resistance for general non acidic environments and various applications.
- Aluminum Alloy 7075: This aluminum alloy shows remarkable anti-rust characteristics and provides high resilience against stress corrosion cracking. It is commonly used in sectors such as aerospace, high-strength structures, and sports equipment.
- Aluminum Alloy 5083: This aluminum alloy shows excellent anti-rust characteristics, particularly in marine environments. It is generally used in many kinds of areas including shipbuilding, marine structures, and offshore engineering.

Summary
Preventing aluminum plate oxidation is essential to preserve their appearance, performance, and structural integrity. By implementing the six methods discussed in this blog post – protective coatings, anodizing, passivation, protective films, proper storage, and avoiding contact with reacting substances – you can effectively protect your aluminum surfaces from oxidation. Remember to choose the most suitable method based on your specific requirements and consult professionals when necessary. With proactive preventive measures, your aluminum plates will maintain their shine and durability for years to come.
FAQ
How quickly does aluminum corrode?
The rate of aluminum corrosion depends on various factors such as the environment, the presence of other metals, and the type of aluminum alloy. In general, pure aluminum alloys have an average corrosion rate of 0.03-0.04 mm/year.
Does aluminum rust easily?
Aluminum does not rust, but it can corrode. When aluminum is exposed to air, it forms a thin layer of aluminum oxide that protects the metal from further oxidation. This oxide layer is hard and serves as a protective coating around the underlying aluminum. It doesn’t flake off like iron oxide and therefore, once the outer layer of aluminum “rusts” the process stops and the aluminum is protected.